Evaluating Oxivir® Tb Versus Criteria of Ideal Disinfectants – HOW DOES IT STACK UP?

Recent studies have demonstrated that the environment plays a role in the transmission of healthcare-associated infections (HAIs). Environmental surfaces and patient care equipment are important reservoirs for pathogens. Contaminated surfaces increase the risk of transmission to patients either through their direct contact with contaminated surfaces or by contaminating the hands of healthcare workers who then transfer these pathogens to patients or other surfaces. This evidence supports the need to improve cleaning and disinfection of environmental surfaces and patient care equipment.
The selection of the right disinfectant is one of the two essential components for effective disinfection. The other component, the practice, relates to the proper application of disinfectant onto surfaces, the proper training of users, and the adherence to manufacturer’s label instructions. The combination of product and practice results in effective surface disinfection, which reduces patient risk and improves patient outcomes.
Since healthcare facilities prefer to use evidence on which to base their decisions and practices, it is important to outline key decision criteria that can be used to select the optimal disinfectant for a facility. Recently, Drs. Rutala and Weber published guidance considerations in the Infection Control and Hospital Epidemiology (Vol. 35, No. 7 (July 2014), pp. 855-865), for healthcare facilities to use when selecting a disinfectant.
- Kill claims for the most prevalent healthcare pathogens
- Fast kill times and acceptable wet-contact time to ensure proper disinfection of noncritical surfaces and patient care equipment
- Safety
- Ease of Use
- Other Factors such as training and support provided by manufacturers, and total cost
This paper will evaluate how Accelerated Hydrogen Peroxide®, specifically Oxivir® Tb, performs against these key criteria and will highlight how it differs from other disinfectant technologies.
Accelerated Hydrogen Peroxide (AHP®) is a synergistic blend of commonly used, safe ingredients that when combined with low levels of hydrogen peroxide, produce dramatically increased germicidal potency and superior cleaning performance. AHP was designed to be tough on pathogens, in short contact times while being gentle on staff, patients, and facility assets.
The benefits of AHP have been validated by third party clinical studies conducted by scientific organizations and third party researchers that are recognized by government regulatory agencies in Europe, Canada, and the United States. AHP has received approvals from the European Union, Health Canada and the United States Environmental Protection Agency (EPA). Several peer-reviewed studies have been published on AHP-based formulas, and many of these are referenced in the index.
1. Kill claims for the most prevalent healthcare pathogens
Oxivir Tb is effective against key healthcare-associated pathogens, including viruses and bacteria, in just 60 seconds. The one-minute efficacy claims include hard-to-kill, small non-enveloped viruses such as norovirus and poliovirus, as well as blood-borne pathogens (e.g. HIV, HBV, and HCV). The chemistry has also proven to be effective in the presence of soil and hard water.
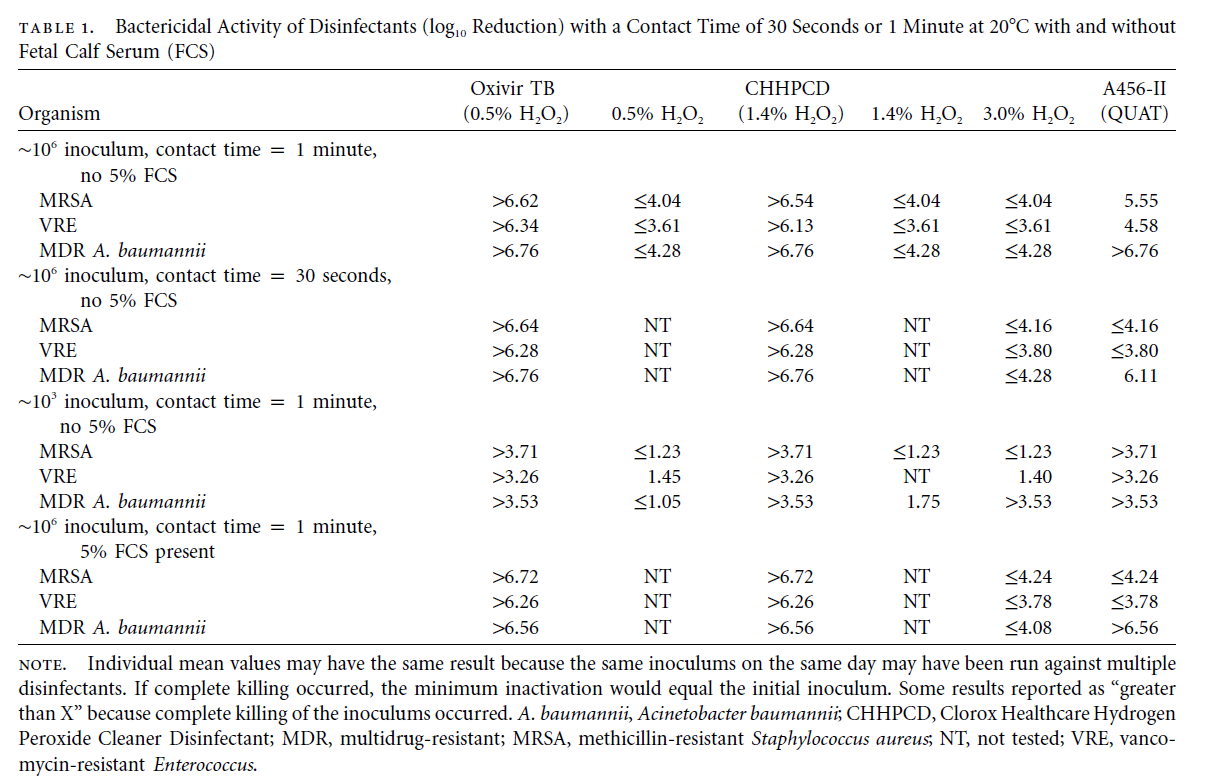
Oxivir Tb demonstrates the same effectiveness with just 0.5% active ingredients, as compared to other hydrogen peroxide-based disinfectants containing almost three times the level of actives to perform at the same level (See Table 1). Higher levels of actives have been known to increase safety risks, and can increase the residue left on surfaces which may cause increased streaking or leave a film.
A soon-to-be published study will also demonstrate its ability to reduce HAI rates across a broad range of pathogens (more information will be coming soon).
2. Fast kill times and acceptable wet-contact time to ensure proper disinfection of noncritical surfaces and patient care equipment
Ideally contact times should be greater or equal to the kill time, or the time listed on the EPA label to achieve disinfection. Wet contact time is a critical component of the disinfectant evaluation because if a product evaporates too quickly, it will not remain in contact with the microorganism for the necessary kill/contact time. Fast kill times are important because they provide confidence that pathogens are killed before the disinfecting solution can dry or be removed. A study published in the Journal of AOAC International (Vol. 93 No 6, p. 1-8), investigated the impact of dry time on product efficacy. Of the six (6) disinfectant products tested, Oxivir Tb was the only chemistry that was able to achieve disinfection using the drying time regardless of contact time, highlighting the fact that facilities that do not achieve the appropriate contact time in accordance with the approved product label may not be achieving the level of kill needed to minimize the risk of transmitting HAIs.
Ensuring that staff is compliant with cleaning and disinfection protocol can reduce the risk of HAIs and re-admissions. One of the key benefits realized with Oxivir Tb is faster and more consistent contact times for a broad spectrum of pathogens. Unlike many disinfectants, Oxivir Tb actually stays wet for its entire contact time (60 seconds), ensuring the disinfection can occur with just one application.
3. Safety
A 2010 multistate report by the CDC1 highlighted the magnitude of acute antimicrobial pesticide illness among workers in healthcare facilities, including the nature and frequency of these exposures. Below is a recap of the report:
- Identified 401 cases of work-related illness associated with antimicrobial pesticide exposures in healthcare facilities
- The chemicals responsible for most healthcare facility cases were quaternary ammonium compounds (quats), glutaraldehyde, and sodium hypochlorite (bleach) which can cause irritant symptoms involving eyes, skin and respiratory tract
- Environmental services staff (24%), nursing (16%) and technicians (15%) were the most frequent occupations reported
- Ocular symptoms (eye irritation/pain) were the most commonly experienced adverse health effect – 55%; neurologic (headaches, dizziness) – 32%; respiratory irritation – 30%; dermal (skin irritation, rash) – 24%
- Most ocular symptoms were frequently associated from splashes while not wearing eye protection (only 15% reported using eye protection)
It is estimated that the average cost per claim2 for eye injuries is $118,000, for neurological injuries is $85,000 and for respiratory injuries is 64,000. If these cost estimates are applied to the report noted above, the cost for these claims for only these 4 states is estimated at $45 million.
1 Acute Antimicrobial Pesticide-Related Illnesses Among Workers in Health-Care Facilities – California, Louisiana, Michigan and Texas 2002-2007, CDC Weekly Morbidity and Mortality Report, May 14, 2010
2 OSHA Safety Pays Program website Acute Antimicrobial Pesticide-Related Illnesses Among Workers in Health-Care Facilities — California, Louisiana, Michigan, and Texas, 2002—2007, MMWR May 14, 2010 / 59(18);551-556; Arif et al Occupational exposures and asthma among nursing professionals Occup Environ Med. doi:10.1136/oem.2008.042382
The report highlighted the need to implement less hazardous antimicrobial pesticide products and, where required, to ensure that the appropriate personal protective equipment (PPE) is conveniently-located and used.
While highly effective against key pathogens, Oxivir Tb was formulated with the comfort, safety, and well-being of staff and patients in mind. In all six EPA toxicity categories, Oxivir Tb falls into Category IV, the lowest level of hazard (practically non-toxic, not an irritant), and requires no safety warnings or PPE. This means that staff will not have to compromise safety for efficacy. By enabling the use of the product with lower risk, staff may be more willing to use it, which facilitates more consistent use, while reducing the risk of worker related injuries and the cost of PPE. Ensuring that staff is compliant with cleaning and disinfection protocol reduces your risk of HAIs and readmissions.
4. Ease of Use
- The easier a product is to use, the more likely the achievement of use compliance. To facilitate use, disinfectants should be available in multiple and convenient forms, and should be composed of a durable substrate that will not easily tear, fall apart, or dry out quickly.
Oxivir Tb is available in a ready-to-use liquid format along with three sizes of wipes – 6” x 7”, 7” x 8” and 11” x 12”. A variety of brackets and stands are available to ensure that the wipes are easily accessible when and where they are needed.
5. Other Considerations
When selecting a disinfectant, it is important to look at the overall cost vs. the just the cost of the disinfectant solution. This calculation should include a variety of factors including labor time to set up and use the product, laundering costs (if applicable), and the cost of non-compliance (i.e. not disinfecting due to safety concerns, inaccessibility or lack of time), and the risks and costs associated with increased HAIs.
Oxivir Tb offers you a simple and compliant solution with broad efficacy along with fast and realistic contact times, an improved safety profile, and easy to use formats. Facilities value the reduction in labor, employee concern, and infection rates.
In summary, Oxivir Tb offers many of the benefits referenced in the ideal disinfectant criteria. These hydrogen peroxide-based disinfectants, powered by AHP technology, can disinfect against most common, and emerging, healthcare-associated pathogens, including multi-drug-resistant organisms, in just 60 seconds. Oxivir Tb offers fast disinfection and superior cleaning ability while remaining gentle for staff, patients and surfaces. They are available in a RTU format along with 3 sizes of wipes, and have accessories available to enable convenience for staff.

